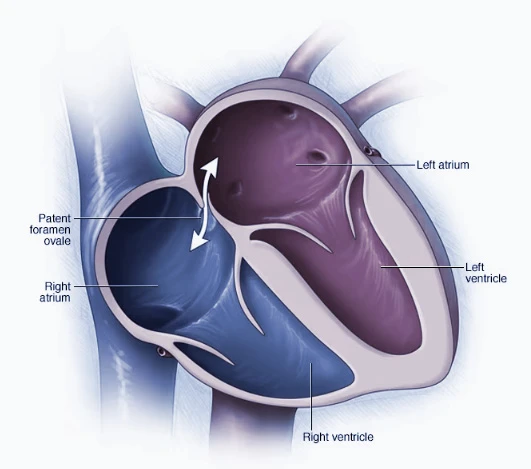
Patent Foramen Ovale (PFO) & Catheter-based Procedures
Catheter-based procedures play a vital role in both diagnosing and treating heart-related issues. In the context of patent foramen ovale (PFO), these procedures involve the use of a catheter to guide the placement of a closure device that permanently seals the hole in the heart wall, preventing abnormal blood flow between atrial chambers.
Overview
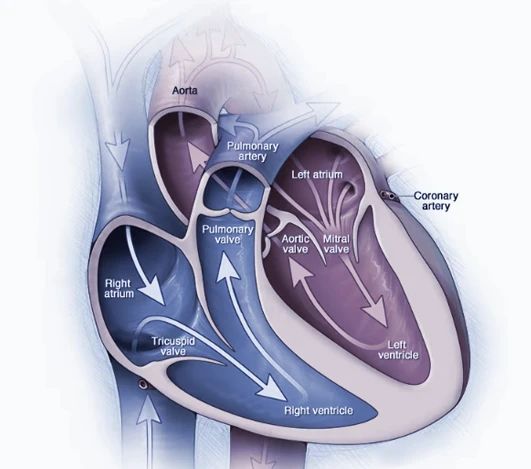
A cardiac catheterization procedure is employed to slowly insert a catheter into the heart through a vein in the inner thigh. Various tests are conducted to measure the PFO and ensure there are no other defects. Angiography and intra-cardiac echo (ICE) imaging help visualize the heart and determine the size of the closure device needed.
Procedure Details
The PFO closure device is guided to the heart through the vein, positioned at the heart wall defect, and formed to straddle each side of the hole. The device, such as the Amplatzer® PFO occluder or GORE® CARDIOFORM Septal Occluder, remains permanently in the heart, preventing abnormal blood flow. The entire catheter-based procedure typically takes one to two hours.
Device Types
FDA-approved devices like the Amplatzer® PFO occluder and others are used for PFO closure. These devices, made of materials with a proven safety record, help eliminate flow across the hole and become integrated into the patient's heart tissue over time.
Comparisons with Medication
Clinical trials comparing catheter-based PFO closure with medication (aspirin or warfarin) show varying results. While some trials reveal no clear difference, others suggest a lower rate of new strokes in patients undergoing PFO closure along with blood-thinning drugs. Further trials are ongoing to explore this comparison.


Follow-up and Home Care
Post-procedure, tests including X-ray, electrocardiogram, and echocardiogram are conducted within 24 hours to ensure device positioning. Patients may be instructed to rest in bed for six hours and are given home care instructions, including medication regimens and restrictions on lifting. Follow-up visits are scheduled at one month, six months, and one year.
Risks / Benefits
The closure devices' materials have a proven safety history, and the body typically reacts positively, with tissue growing over the device within a few days. The patient will not feel the device, and it won't be affected by security sensors or household appliances. Some reduction in MRI or CT image clarity may occur due to the wire frame on occluder devices.